
"Wednesday Oct. 9th " 5 PM EST
Register for Tom's DITAWorld 2019 Session
"How the FDA uses DITA to author and edit the Food Code”
Some of us like instant gratification. Others like to analyze, test and implement in a controlled environment in order to ensure that the success of our undertaking is long lasting, and can be replicated. For many companies, constant tribulation spurs change. One arduous task in a series of necessary steps prevents them from moving forward in a meaningful way, leading to the old adage “two steps forward, one step back”. In tech comm, this problem usually manifests itself in the form of multiple writers creating unstructured content with a loose set of corporate style guides that tend to only focus on the formatting requirements. With that lack of control over the source content, they are leaving themselves open to human error, either through corrupting the content, poor revision management, or not fully adhering to the style guide.

One organization that found themselves falling victim to this pattern was the U.S. Food and Drug Administration (FDA). As many people already know, this branch of the federal government focuses on the safe handling and preparation of food and pharmaceuticals, and the steps to implement the recommended practices. For their first migration project, they wanted to take the latest version of the Food Code, originally created in MS Word, and convert it to XML. In the past, multiple content contributors would funnel their new or edited content through a review PDF process where a team would compile any edits, and make final adjustments before publishing. They wanted to be able to easily manage their book from Frame, and efficiently add, remove and edit content without disrupting the production and print schedule of the book. The book is printed, and published to PDF so that it can be passed to the thousands of smaller state level food and drug regulators all over the U.S.
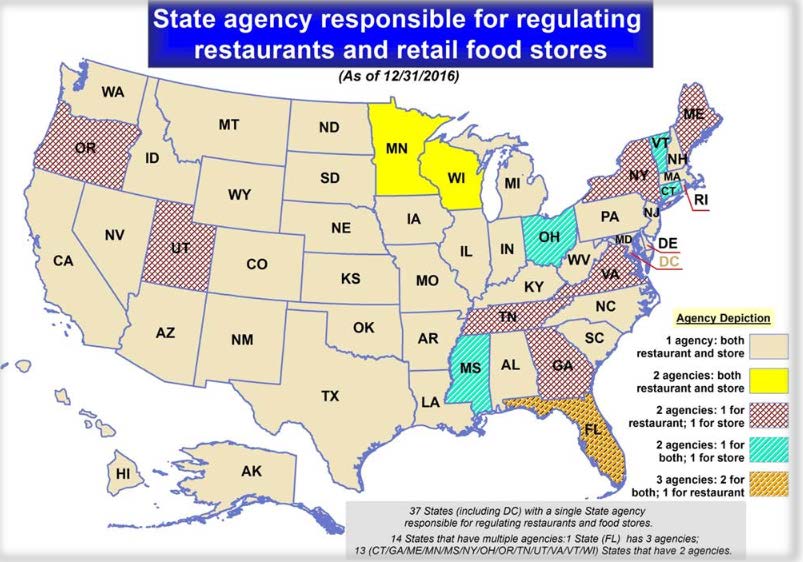
Time consumption was a major issue for them, and probably one of the worst killers of their productivity. So, instead of giving free reign over the content, the FDA chose to move forward with a new content strategy for the Food code that would give them greater control over the source content and reduce the time to market. That reduction in time to produce the Food Code was the driving factor in their quest for a better work flow and is something that a standard like DITA would allow them to accomplish.
The FDA sought to remedy all of their issues by implementing a new work flow. They just weren’t sure which way to go. So, they turned to The Content Era and Tom Aldous for advice. They learned that the migration can be easily accomplished in FrameMaker and that they shouldn’t be worried about disrupting the ongoing development of the Food Code. He then showed them DITA. DITA is “pre-loaded” with a logical subset of elements to develop with, can be specialized if needed, and can be mapped to fairly easily using Adobe FrameMaker. Being a leader in DITA functionality, FM can set DITA as the structured application so that DITA content can flow through a custom FM template that adds the formatting automatically. DITA/FM gives them the desired control over user error, bundling tasks to “one-click”, and the ability to automate tasks because of it's compatibility with XSL, reducing their time to market. They were smitten.
The Content Era has a long standing history with DITA/XML content. Tom, the CEO of TCE, has been helping major companies and government entities convert to XML and DITA for many years. So, it was an easy decision for the FDA to take his advice when it came to migrating and trusted his guidance as they set forth on implementing the changes. Tom is a leader in DITA/XML implementation, especially in Adobe FrameMaker. His experience comes from a culmination of 30 years of work in the trenches, designing content landscapes, successfully; first, as the co-founder and president of Integrated Technologies, then as the Director of Tech Comm Business Development for Adobe Systems.
With an outstanding reputation, and coming off of a gig as Senior VP of Global Ops for Acrolinx, a german AI software company, Tom set out to do something more. He wanted to build a company that provided Tech Comm support and implementation services for the US government as a Schedule 70 certified US GSA Vendor. That way, he could have a part in making the Federal Government run more efficiently. So, he started The Content Era.
In a short amount of time, TCE accomplished what others weren’t able to by providing knowledge and the expertise that would guide the GSA to a successful phase 1 project completion without disrupting their current work flow. Today, the GSA continues to lean on them for any and all new content quandaries and will look to them for guidance throughout future phases. That same understanding of how the government operates is what made TCE a perfect fit for the FDA DITA migration project. They decided to move forward, with little persuasion.
They started their journey by migrating the content to DITA using the baked in MS Word style tags and converting them to DITA elements using an FM conversion table. Once the content was mapped it was time to break up the content into a ditamap so that each section, chapter and annex was it’s own topic reference that called out a specific section of the Food Code. In the past, things like TOC’s and indexes were manually entered into the book. This presented an opportunity for TCE to use the power of Frame to create a composite book that was comprised of DITA topics from the ditamap, and FM generated files like the Table of Contents and Index. This would make the files dynamic and would update automatically anytime a book update was done.
Another way they made the book dynamic was by applying the numbering to each chapter and annex (and all the subsequent nested topics and concepts) from the book’s numbering scheme - another benefit of the “update book” command. This ensured that the individual chapter/annex numbering, xrefs and sub-section TOC’s were all properly numbered after any additions to the book. This was exactly the control they were looking for as it reduced the amount of manual steps to make changes to the book. Plus, there was the default navigation improvement that happens anytime you use a ditamap or composite FM book.
Next it was time to start programming the EDD to automatically apply the formatting to the content that would match the look and feel of the MS Word version of the Food Code. This is where FM shines bright. There isn’t much you CAN’T do in an Adobe FM EDD. It’s ability to provide a visual representation of the final published document, and edit against it, is unmatched by any other XML editor. TCE used the EDD to create a guided authoring environment that would restrict users from making mistakes and tampering with the formatting. Now, the DITA content flows through the FM template and opens up with all the styles they use in the Food Code, automatically.
Finally, the team was ready to learn how to use their new Food Code editing environment. A couple of administrator and template training sessions as well as end user training would complete their migration to DITA. Now, they are ready to print the Food Code book for distribution, as well as provide the online PDF version of the content, in a fraction of the time. Normally, an iteration of the Food Code would take years to edit and publish. Now, with the help of DITA/FrameMaker they’ve reduced the turn around time to just a few months. And when they are ready, they can use the same model to convert all the other important FDA documents to DITA as well. Making their content library smarter and versatile to grow with the addition of any government regulations.
Print and PDF are just the beginning. Because they are in FM, the FDA team can swiftly change the Food Code and re-publish/distribute AND publish to multiple formats in order to deliver the content faster to a broader audience. Publishing to basic HTML, HTML5 and ePub out of the box with FrameMaker gives them a dynamic web-page and e-reader output that prepares them for a future Food Code that is edited, managed, and delivered through a web browser on an array of devices. Plus, FrameMaker is compatible with many DITA Component Content Management Systems that allow for single sourcing and automatic content delivery among many other benefits. When lives are at stake, it's vital to deliver the most accurate content as fast as possible. Today, the FDA is capable of doing that by making the switch from MS Word to DITA/FM. Tomorrow, they’ll be able to sustain that demand and manage their content more efficiently with DITA/FM.




